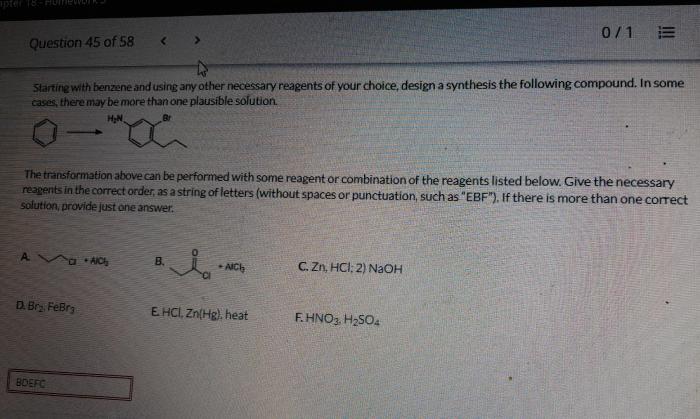
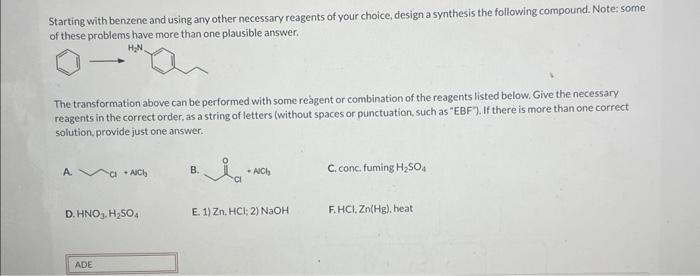
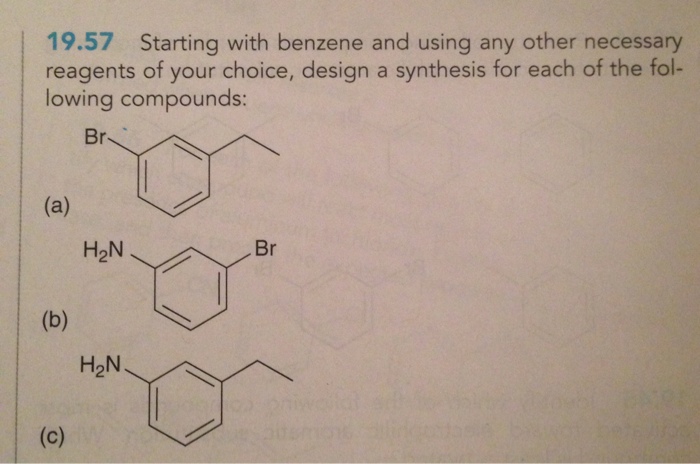
Starting with benzene and using any other necessary reagents – Embarking on a journey of organic synthesis, we commence with the versatile benzene molecule and an array of indispensable reagents. This comprehensive guide delves into the captivating world of electrophilic and nucleophilic aromatic substitution reactions, oxidation and reduction processes, and the intricacies of Friedel-Crafts and Diels-Alder reactions.
As we unravel the mechanisms and regioselectivity governing these transformations, a deeper understanding of benzene chemistry unfolds, empowering synthetic chemists with a formidable arsenal for molecular construction.
Benzene Ring Structure and Properties: Starting With Benzene And Using Any Other Necessary Reagents
Benzene is an aromatic hydrocarbon with the chemical formula C 6H 6. It is a colorless, flammable liquid with a distinctive odor. Benzene is a major component of gasoline and is used in the production of many other chemicals.
The benzene molecule has a hexagonal ring structure with alternating single and double bonds. This structure gives benzene its characteristic resonance and aromaticity. Resonance means that the electrons in the benzene ring are delocalized, which makes the molecule more stable.
Aromaticity means that the benzene ring is planar and has a closed shell of electrons, which makes it resistant to electrophilic attack.
Benzene is a relatively unreactive compound, but it can undergo a variety of reactions, including electrophilic aromatic substitution, nucleophilic aromatic substitution, oxidation, and reduction.
Electrophilic Aromatic Substitution Reactions
Electrophilic aromatic substitution reactions are reactions in which an electrophile (a positively charged species) attacks a benzene ring and replaces one of the hydrogen atoms. The most common electrophile is the nitronium ion (NO 2+), which is formed from nitric acid and sulfuric acid.
The mechanism of electrophilic aromatic substitution reactions involves the following steps:
- The electrophile attacks the benzene ring and forms a Wheland intermediate.
- The Wheland intermediate rearranges to form a more stable intermediate.
- The proton is removed from the more stable intermediate to form the product.
The regioselectivity and orientation of electrophilic aromatic substitution reactions are determined by the nature of the electrophile and the substituents on the benzene ring.
Nucleophilic Aromatic Substitution Reactions
Nucleophilic aromatic substitution reactions are reactions in which a nucleophile (a negatively charged species) attacks a benzene ring and replaces one of the hydrogen atoms. The most common nucleophile is the hydroxide ion (OH –), which is formed from water.
The mechanism of nucleophilic aromatic substitution reactions involves the following steps:
- The nucleophile attacks the benzene ring and forms a Meisenheimer complex.
- The Meisenheimer complex rearranges to form a more stable intermediate.
- The proton is removed from the more stable intermediate to form the product.
The regioselectivity and orientation of nucleophilic aromatic substitution reactions are determined by the nature of the nucleophile and the substituents on the benzene ring.
Oxidation and Reduction Reactions of Benzene, Starting with benzene and using any other necessary reagents
Benzene can be oxidized to form phenol (C 6H 5OH) or aniline (C 6H 5NH 2). The oxidation of benzene to phenol is catalyzed by potassium permanganate (KMnO 4), while the oxidation of benzene to aniline is catalyzed by chromic acid (H 2CrO 4).
Benzene can be reduced to form cyclohexane (C 6H 12). The reduction of benzene to cyclohexane is catalyzed by hydrogen gas (H 2) and a nickel catalyst.
Key Questions Answered
What is the significance of benzene’s resonance and aromaticity?
Benzene’s resonance and aromaticity endow it with exceptional stability and unique chemical properties, making it a versatile building block for a wide range of organic compounds.
How do electrophiles and nucleophiles influence electrophilic and nucleophilic aromatic substitution reactions?
Electrophiles and nucleophiles play crucial roles in these reactions, with electrophiles seeking electron-rich sites on the benzene ring and nucleophiles targeting electron-deficient positions.
What factors govern the regioselectivity and orientation of Friedel-Crafts reactions?
The regioselectivity and orientation of Friedel-Crafts reactions are influenced by the steric and electronic effects of the substituents on the benzene ring and the nature of the electrophile.