Embark on a captivating journey with our Drawing Lewis Structures Practice Worksheet, an invaluable tool for honing your skills in visualizing chemical bonding. Dive into the fascinating world of Lewis structures, unraveling their secrets and unlocking a deeper understanding of molecular interactions.
As you delve into this practice worksheet, you’ll discover the fundamental concepts of Lewis structures, gaining insights into their construction and significance. Engage in a series of carefully crafted exercises, testing your comprehension and expanding your knowledge of chemical bonding.
Prepare to elevate your chemistry prowess and unlock the mysteries of molecular architecture.
1. Basic Concepts: Drawing Lewis Structures Practice Worksheet
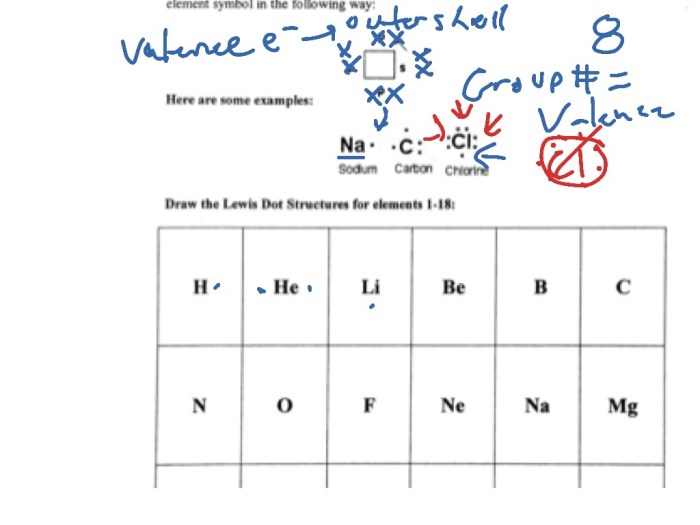
Lewis structures are a way of representing the bonding between atoms in a molecule. They show the arrangement of electrons in the molecule and can be used to predict the shape of the molecule and its chemical properties.
To draw a Lewis structure, you need to know the number of valence electrons in the molecule. The valence electrons are the electrons in the outermost shell of the atoms.
Once you know the number of valence electrons, you can start to draw the Lewis structure. The first step is to connect the atoms together with single bonds. A single bond is a bond between two atoms that share two electrons.
After you have connected the atoms with single bonds, you can add double bonds or triple bonds. A double bond is a bond between two atoms that share four electrons. A triple bond is a bond between two atoms that share six electrons.
The final step is to add lone pairs of electrons. Lone pairs are electrons that are not involved in any bonds.
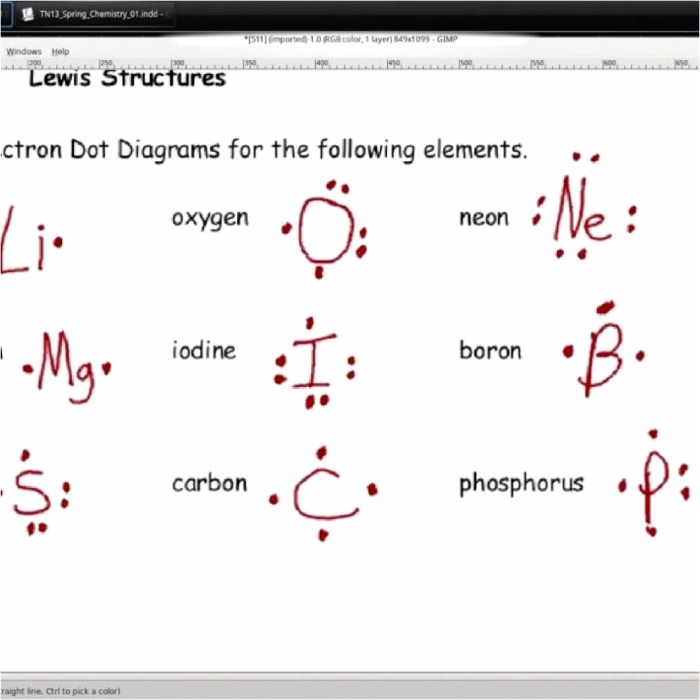
Examples of Lewis Structures
Here are some examples of Lewis structures:
- Water (H2O): H:O:H
- Carbon dioxide (CO2): O=C=O
- Methane (CH4): H:C:H | H:C:H
- Ammonia (NH3): H:N:H | H
Rules for Drawing Lewis Structures, Drawing lewis structures practice worksheet
There are a few rules that you need to follow when drawing Lewis structures:
- The total number of electrons in the Lewis structure must equal the number of valence electrons in the molecule.
- Each atom must have a complete octet of electrons. An octet is eight electrons.
- Hydrogen atoms can only have two electrons.
- Lone pairs of electrons can only be placed on atoms that have a lone pair of electrons in their valence shell.
FAQ Compilation
What is the significance of Lewis structures?
Lewis structures provide a visual representation of electron distribution within molecules, offering insights into their bonding patterns, molecular shapes, and chemical properties.
How can I determine the number of valence electrons in a molecule?
To determine the number of valence electrons, sum the valence electrons of each atom in the molecule. For example, in water (H2O), each hydrogen atom contributes one valence electron, resulting in a total of two valence electrons. The oxygen atom contributes six valence electrons, bringing the total to eight valence electrons for the water molecule.
What are the key rules for drawing Lewis structures?
The key rules for drawing Lewis structures include:
- Determine the total number of valence electrons.
- Connect atoms with single bonds to satisfy their valence.
- Add lone pairs of electrons to complete octets (except for hydrogen).
- Adjust the bonding to minimize formal charges.